Introduction: Axi-cel utilization expanded after demonstration of superior event-free survival in patients (pts) with early relapsed or primary refractory LBCL (Locke, et al. NEJM. 2022) and was reinforced by improved overall survival (OS) among these pts (Westin, et al. NEJM. 2023). Despite increased demand, limited data exist on the impact of bridging therapies (BT) on pt outcomes. Thus, BT use between leukapheresis and lymphodepletion (LD) is largely at the discretion of treating physicians. Here, we assessed the impact of BT on effectiveness and safety outcomes after axi-cel.
Methods: Pts receiving axi-cel for 3L+ LBCL in the US between 2017-2020 were prospectively collected through the CIBMTR registry in a post-authorization safety study; data up to May 2023 were included. Effectiveness outcomes were overall response rate (ORR), complete response (CR) rate, duration of response (DOR), progression-free survival (PFS), and OS. Safety outcomes were cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) per ASTCT grading, non-relapse mortality (NRM), and other key adverse events. Propensity score-based inverse probability of treatment weighting (IPTW) was used in logistic and Cox regressions to account for potential confounding in BT use. For pts who had BT, effects of BT types and responses on outcomes were evaluated using conventional logistic and Cox regressions.
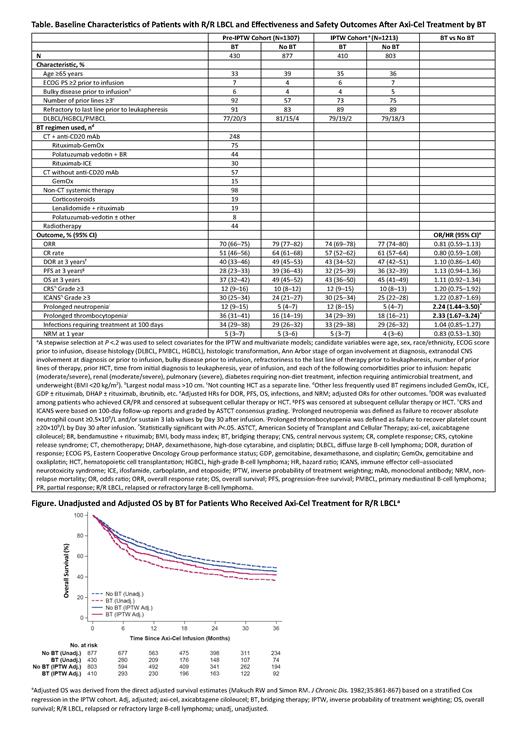
Results: Of 1497 pts registered, 1307 had BT data available and were included (median follow-up, 36 mo). Overall, 430 pts (33%) received BT (median time from leukapheresis to LD, 23 days [IQR, 21-28]). Systemic therapy was used in 403 pts (31%), including 305 (23%) with chemotherapy (CT)-based BT (19% treated with CT + anti-CD20 monoclonal antibody [mAb; mainly rituximab] and 4% with CT only), and 98 (7%) with non-CT systemic therapy (Table). Radiotherapy (± systemic therapy) was used in 44 pts (3%). Pts with ≥3 lines of prior therapy (odds ratio [OR], 10.67 [95% CI, 7.17−15.87]) or mild hepatic impairment prior to infusion (OR, 1.64 [1.06−2.55]) were more likely to receive BT, while those with histologic transformation (OR, 0.69 [0.51−0.92]) or longer time from diagnosis to leukapheresis were less likely to receive BT.
ORRs to axi-cel were 70% and 79% (CR rates 51% and 64%) for pts with or without BT, respectively. Unadjusted DOR, PFS, and OS at 3 years were 40%, 28%, and 37%, respectively, in pts with BT vs 49%, 39%, and 49%, respectively, in those without BT. After multivariate adjustment, no statistical significance was found between BT and any effectiveness outcome. Adjusted hazard ratios (HRs) for BT were 1.13 (95% CI, 0.94-1.36) for PFS and 1.11 (95% CI, 0.92-1.34) for OS, respectively (Figure).
Most safety endpoints were consistent regardless of BT. Grade ≥3 CRS and ICANS occurred in 12% and 30% of pts, respectively, who received BT vs 10% and 24% of pts who did not. Use of BT was associated with more frequent prolonged neutropenia (OR, 2.24 [1.44-3.50]) and thrombocytopenia (OR, 2.33 [1.67-3.24]), but not associated with a higher rate of infections or NRM (Table).
Among pts receiving BT, 74 (17%) had a CR or partial response to BT. Of them, median PFS and median OS post infusion were 12.7 mo (3.8-not estimable [NE]) and 48.7 mo (18.6-NE), respectively. Response to BT was associated with increased CR rate (OR, 2.00 [1.07-3.75]), PFS (HR, 0.69 [0.49-0.99]), and OS (HR, 0.59 [0.40-0.88]) and decreased any-grade ICANS (OR, 0.51 [0.29-0.92]).
When different BT strategies were considered, effectiveness and most safety outcomes for CT + anti-CD20 mAb vs non-CT treatment were comparable without significant difference. CT + anti-CD20 mAb was associated with improved ORR (OR, 1.90 [1.00-3.60]), PFS (HR, 0.69 [0.49-0.98]), and OS (HR, 0.66 [0.46-0.95]) but increased any-grade CRS (OR, 2.16 [1.02-4.57]) vs CT only. CT use was associated with an increased risk of prolonged thrombocytopenia (41% vs 24% for pts receiving non-CT treatment; OR, 2.37 [1.39-4.02]). No significant association was found between use of radiotherapy as BT and any outcome.
Conclusion: These US real-world findings suggest that the addition of BT prior to axi-cel is associated with more frequent prolonged cytopenias but does not impact effectiveness or other critical safety outcomes. Response to BT may prognosticate more favorable outcomes after axi-cel infusion. Further study is warranted to identify the optimal bridging regimen.
Disclosures
Cook:Curio Science, a Vaniam Group Network: Honoraria. Perales:Celgene: Honoraria; Sellas Life Sciences: Consultancy; Adicet: Honoraria; Incyte: Consultancy, Honoraria, Research Funding; Syncopation: Honoraria; Equillium: Consultancy, Honoraria; Vor Biopharma: Consultancy, Honoraria; NexImmune: Consultancy, Current equity holder in publicly-traded company; Kite: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Exevir: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Miltenyi Biotec: Honoraria; Caribou: Consultancy, Honoraria; Nektar Therapeutics: Consultancy, Honoraria, Research Funding; Allovir: Consultancy; Allogene: Research Funding; AbbVie: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Cidara Therapeutics: Consultancy, Other; Novartis: Consultancy, Honoraria, Research Funding; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; Omeros: Consultancy, Current equity holder in publicly-traded company, Honoraria; Orcabio: Consultancy, Current equity holder in publicly-traded company, Honoraria; Servier: Other; DSMB: Other; Medigene: Consultancy, Other; MorphoSys: Consultancy, Honoraria; VectivBio AG: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Dahiya:Kite, a Gilead Company: Consultancy, Research Funding; Adaptive Biotechnologies: Consultancy; Bristol Myers Squibb: Consultancy; Incyte: Consultancy. Nastoupil:Caribou Biosciences: Honoraria, Research Funding; Gilead Sciences/Kite Pharma: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; AstraZeneca: Honoraria; DeNovo: Honoraria; ADC Therapeutics: Honoraria; Regeneron: Honoraria; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; AbbVie: Honoraria. Jacobson:Daiichi-Sankyo: Consultancy; ImmPACT Bio: Consultancy; Instil Bio: Consultancy; Caribou Bio: Consultancy; Abintus Bio: Consultancy; AstraZeneca: Consultancy; ADC Therapeutics: Consultancy; Abbvie: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Novartis: Consultancy; Kite, a Gilead company: Consultancy, Research Funding; Morphosys: Consultancy; Ipsen: Consultancy; Miltenyi Biotec: Consultancy; Synthekine: Consultancy; Pfizer: Research Funding. Budde:Amgen: Research Funding; Roche: Consultancy; Novartis, Gilead, F. Hoffmann-La Roche Ltd, BeiGene, Genentech, Inc.: Consultancy; ADC Therapeutics: Consultancy; AstraZeneca: Consultancy, Research Funding; Merck: Research Funding; MustangBio: Research Funding. Logan:Enlivex: Consultancy. Hu:Gilead Sciences: Current holder of stock options in a privately-held company; Kite, a Gilead Company: Current Employment. Wang:Kite, a Gilead Company: Current Employment. Luo:Kite, a Gilead Company: Current Employment. Kim:Gilead Sciences: Current holder of stock options in a privately-held company; Kite, a Gilead Company: Current Employment. Smith:Gilead Sciences: Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company; Kite, a Gilead Company: Current Employment. Lee:Gilead: Current holder of stock options in a privately-held company, Other; Kite, a Gilead Company: Current Employment, Other: Travel expenses . Sun:Gilead Sciences: Current holder of stock options in a privately-held company, Other; Kite, a Gilead Company: Current Employment. Pasquini:Janssen: Research Funding; Novartis: Research Funding; Kite, a Gilead Company: Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Kite Brazil: Honoraria. Locke:Kite, a Gilead Company: Consultancy, Other: Institutional ; Emerging Therapy Solutions: Consultancy; Cellular Biomedicine Group: Consultancy; Novartis: Consultancy, Other: Institutional ; Janssen: Consultancy; Sana: Consultancy; Takeda: Consultancy; Wugen: Consultancy; Caribou: Consultancy; Clinical Care Options Oncology: Other; Imedex: Other; Aptitude Health: Other; Legend Biotech: Consultancy; Iovance: Consultancy; BioPharma Communications CARE Education: Other; ASH: Other; Umoja: Consultancy; CERo Therapeutics: Other: Institutional; Cowen: Consultancy; bluebird bio: Consultancy, Other: Institutional ; A2: Consultancy, Other: Travel support; GammaDelta Therapeutics: Consultancy; Allogene: Consultancy, Other: Institutional ; Amgen: Consultancy; Calibr: Consultancy; BMS/Celgene: Consultancy, Other: Institutional ; Individual Patents: Patents & Royalties: Several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy; Gerson Lehrman Group (GLG): Consultancy; EcoR1: Consultancy; Daiichi Sankyo: Consultancy; National Cancer Institute: Other: Institutional ; Leukemia and Lymphoma Society: Other: Institutional ; Society for Immunotherapy of Cancer: Other: Institutional .

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal